Empowering Research Teams with Knowledge & Compliance
We provide specialized training programs to equip research site staff with the necessary knowledge and skills to conduct clinical trials efficiently while ensuring compliance with regulatory standards. Our goal is to simplify complex research protocols and prepare teams for successful study execution.
Our Training Solutions
- Understanding Research & Protocols – Breaking down complex research protocols into easy-to-understand language, ensuring clarity on study objectives, schedules, and responsibilities.
- Regulatory Compliance & Guidelines – Training on adherence to FDA, IRB, GCP (Good Clinical Practice), ICH (International Council for Harmonisation), and HIPAA (Health Insurance Portability and Accountability Act) regulations to maintain ethical and legal standards.
- Study Preparation & Execution – Teaching teams how to prepare ahead for recruitment and retention, anticipate challenges, and optimize study workflows for smooth trial management.
- Activity & Duty Scheduling – Simplifying the schedule of activities, ensuring staff understand their roles and responsibilities for effective study coordination.
- Building a Strong Research Foundation – Providing essential training on industry advancements, best practices, and evolving regulatory requirements to enhance research site efficiency and quality.

Why Choose Our Training?
- Ensures regulatory compliance and ethical research conduct
- Improves staff confidence in handling clinical trials
- Enhances recruitment and retention strategies
- Simplifies complex research protocols into easy, actionable steps
- Builds a strong foundation for long-term research success
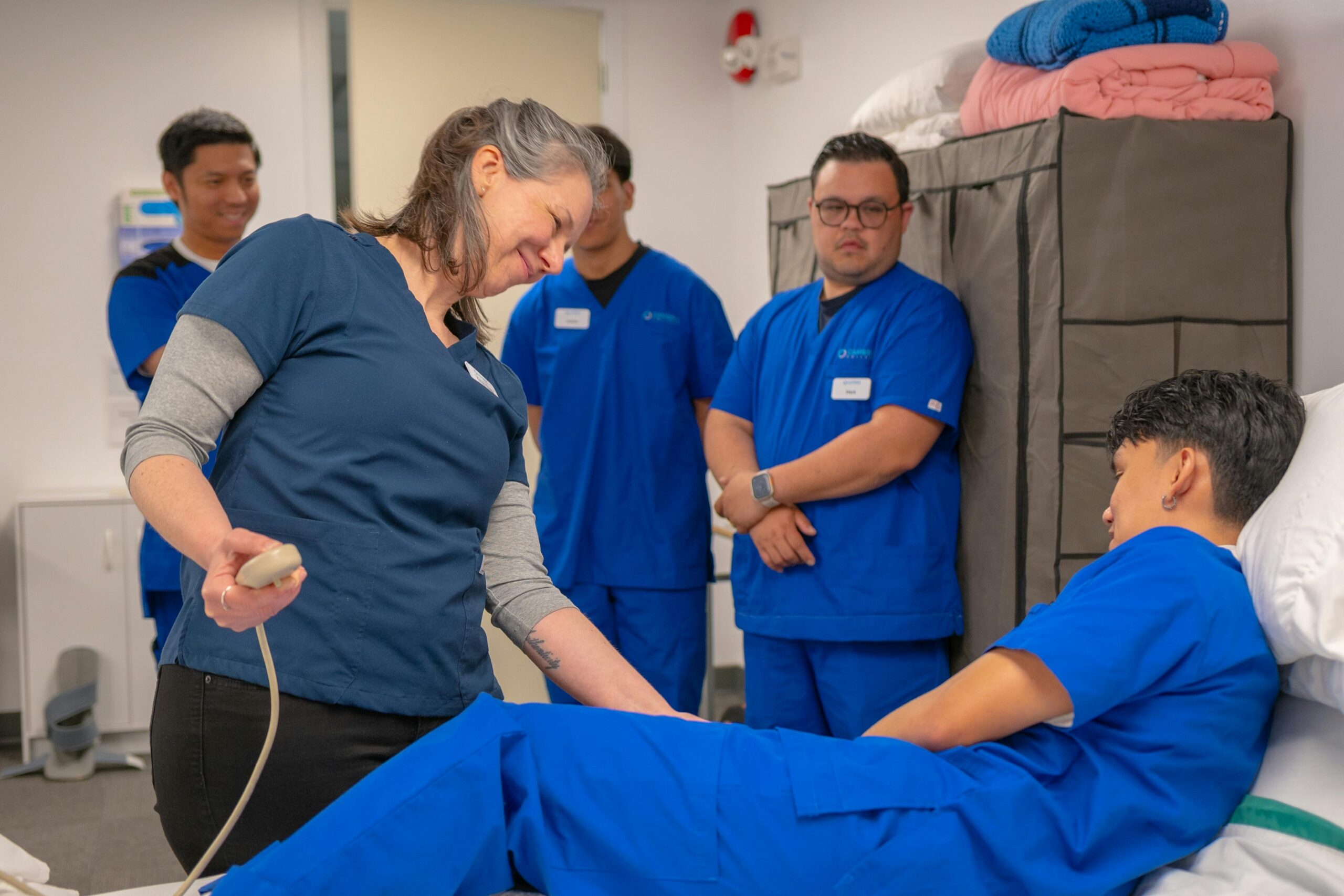
Prepare your research team for excellence—get trained with us today!

